This GARNet research Roundup includes a broad range of topics and contributing institutions. First is a study from TSL that investigates the molecular basis of Arabidopsis and Brassica responses to white rust disease. Second is work from Warwick that uses Arabidopsis as a tool to test genes involved in the evolution of Flax domestication.
The third paper is work from Cambridge that models the response of the circadian oscillator to nicotinamide whilst the fourth paper is a study from the University of Dundee that compares differential gene expression software in the analysis of RNAseq data from a complex organism. The penultimate paper includes a co-author from the University of Oxford and has generated an extended phylogeny of the Brassicaceae family. The final paper compares the growth and metabolite profiles of Arabidopsis and Eutrema salsugineum following drought stress.
Cevik V, Boutrot F, Apel W, Robert-Seilaniantz A, Furzer OJ, Redkar
A, Castel B, Kover PX, Prince DC, Holub EB, Jones JDG (2019) Transgressive segregation reveals mechanisms of Arabidopsis immunity to Brassica-infecting races of white rust (Albugo candida). Proc Natl Acad Sci U S A. doi: 10.1073/pnas.1812911116
Open Access

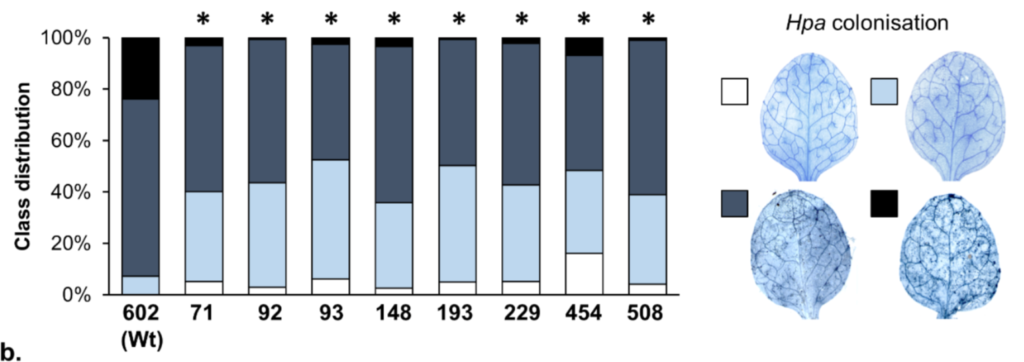
Volkan Cevik is the lead author on this international collaboration that is led by Jonathan Jones at the Sainsbury Lab, Norwich. They have taken advantage of Arabidopsis resistance to white rust (Albugo candida) and used the Multiparent Advanced Generation InterCross (MAGIC) lines to identity the genes responsible for this resistance. This is important as related crop species Brassica juncea and Brassica oleracea are sensitive to this economically important pathogen. They identified a range of nucleotide-binding, leucine-rich repeat (NLR)-encoding genes that were involved in resistance to the pathogen.
Gutaker RM, Zaidem M, Fu YB, Diederichsen A, Smith O, Ware R, Allaby RG (2019) Flax latitudinal adaptation at LuTFL1 altered architecture and promoted fiber production. Sci Rep. doi: 10.1038/s41598-018-37086-5
Open Access

Rafal Gutaker is the lead author on this collaborative study between the University of Warwick and colleagues in Germany, Canada and Denmark, which investigated the route of domestication of the cultivated crop Flax. At northern european latitudes flax evolved to become a fibre crop rather than an oil crop by stem expansion and reduction of seed size. The authors investigated the role in this adaptation of PEBP family genes in the flax genome, LuTFL1 and LuTFL2. LuTFL1 was heterologously expressed in Arabidopsis, demonstrating that it is able to perform roles in flowering time and plant architecture. This research highlights the importance of Arabidopsis as a tool for testing the function of genes from less-easily transformed organisms.
Mombaerts L, Carignano A, Robertson FR, Hearn TJ, Junyang J, Hayden
D, Rutterford Z, Hotta CT, Hubbard KE, Maria MRC, Yuan Y, Hannah MA,
Goncalves J, Webb AAR (2019) Dynamical differential expression (DyDE) reveals the period control mechanisms of the Arabidopsis circadian oscillator. PLoS Comput Biol. doi: 10.1371/journal.pcbi.1006674
Open Access
Laurents Mombarts is the first author in this collaboration between the departments of Plant science and Engineering at the University of Cambridge that looked at the mechanistic effect on nicotinamide on the timing of the circadian oscillation. They developed a systematic and practical modeling framework for the gene regulatory circuits that respond to nicotinamide. They initially developed a mathematical model and then experimentally confirmed their predictions to uncover a role for blue light signalling in this response. Overall their approach could be adapted to predict mechanisms of drug action in other complex biological systems.
Froussios K, Schurch NJ, Mackinnon K, Gierlinski M, Duc C, Simpson GG, Barton GJ (2019) How well do RNA-Seq differential gene expression tools perform in a complex eukaryote? A case study in A. thaliana. Bioinformatics. doi: 10.1093/bioinformatics/btz089
Open Access
Gordon Simpson and colleagues at the University of Dundee collaborate with researchers in Clermont-Ferrand with Kimon Froussios as first author. They use Arabidopsis as a model to test a set of Differential Gene Expression (DGE) tools for the effective analysis of RNAseq data generated with three or fewer biological replicates. They tested nine widely used DGE tools and ultimately recommend the use of tools that are based on the negative binomial distribution.

Nikolov LA, Shushkov P, Nevado B, Gan X, Al-Shehbaz IA, Filatov D, Bailey CD, Tsiantis M (2019) Resolving the backbone of the Brassicaceae phylogeny for investigating trait diversity. New Phytol. doi: 10.1111/nph.15732.

This German, US and UK collaboration is led by Lachezar Nikolov and includes Dmitry Filatov from the University of Oxford as a co-author. They generated a phylogeny of the Brassicaceae, the family that contains Arabidopsis and a number of economically important crops. They used a mixture of fresh tissue and herbarium samples to perform the analysis on almost 80 species; enabling the resolution of new relationships between family members. This work represents an important tool for phylogenetic and comparative studies to maximise future outputs.
Pinheiro C, Dickinson E, Marriott A, Ribeiro IC, Pintó-Marijuan M,
António C, Zarrouk O, Chaves MM, Dodd IC, Munné-Bosch S, Thomas-Oates
J, Wilson J (2019) Distinctive phytohormonal and metabolic profiles of Arabidopsis thaliana and Eutrema salsugineum under similar soil drying. Planta. doi: 10.1007/s00425-019-03095-5
This collaboration between the UK and Portugal is led by Carla Pinheiro and the corresponding author is Julie Wilson from the University of York. Eutrema salsugineum is a stress-tolerance relative of Arabidopsis and in this study the authors have compared the response of these plants following growth on drying soils. Whereas stomatal sensitivity was similar in both species there were significant differences in metabolite profiles and water usage following drought stress. This analysis allowed the authors to conclude that Arabidopsis is indeed a good model for analysis of responses to commonly encountered levels of drought stress.